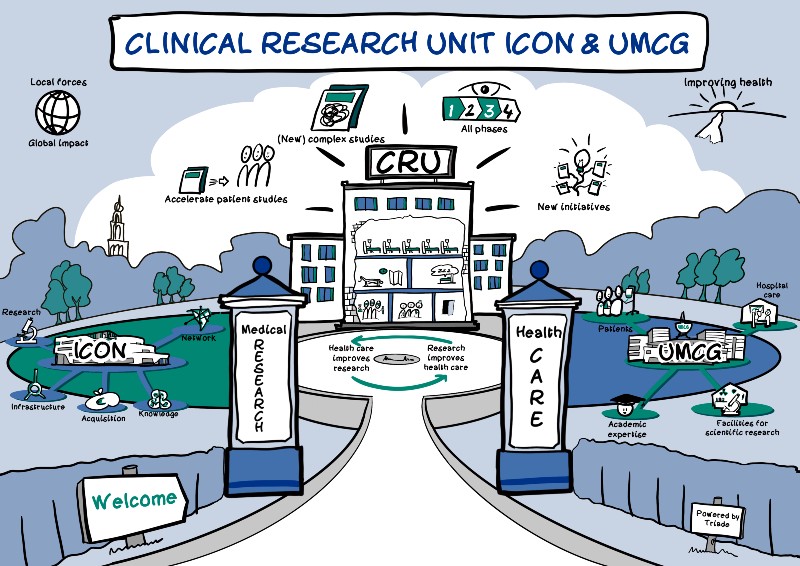
UMCG and ICON collaborate on new clinical research unit in Groningen
In collaboration with ICON, the University Medical Centre Groningen starts a Clinical Research Unit to carry out new research among patients at an early stage. This collaboration, which is unique in the Netherlands, means that critical data and research results for new and existing medications could be available earlier.
The collaboration between the UMCG and ICON in this new Clinical Research Unit makes it possible to carry out early research among patients with various medical conditions who have to stay in a hospital. Medicines can be tested at an early stage, allowing them to be developed more quickly. The Clinical Research Unit will be built on the UMCG site and is expected to be operational in the first quarter of 2022.
Strengthening clinical research
For the UMCG, the collaboration is an important step into the research of new medicines. Dean and Executive Board member of the UMCG Marian Joëls is therefore enthusiastic. “As the UMCG, we want to make progress with treatment through research. We also have the means to provide specialized medical care. The collaboration with ICON gives us access to worldwide networks that are necessary for the recruitment of studies. In addition, ICON has the knowledge and competences required for the design, organisation and implementation of this type of complex study. We herewith increase the quality of early phase studies within our university medical centre. Combining the knowledge and expertise acts as a catalyst for the development of medicines. That is good news for our research and for the patient.”
Faster understanding of the effect of medicines
ICON (formerly PRA Health Sciences), an international healthcare intelligence and clinical research company, has been conducting research in the Netherlands into the effects of drugs and vaccines among healthy participants for more than 35 years, meeting the highest quality standards. According to ICON, the cooperation brings a far-reaching change in the development of medicines. Ronald Koning, VP Clinical Research Services at ICON: “By conducting research among patients at an early stage, we know earlier whether a new drug could work well. This will improve the quality of life and contributes to an effective treatment of diseases where this is currently not possible. We are proud that with this collaboration we can contribute to the complex research questions that UMCG has based on its medical knowledge and academic background.”
Unique collaboration
The collaboration entered into by UMCG and ICON is unique in Europe. The cooperation brings together the necessary expertise and infrastructure of a university medical centre and a renowned research institute in a specially built unit, for which Triade is responsible. Edward van der Meer, director of Triade: “We have recently officially concluded our cooperation. Now we can start building the Clinical Research Unit.”
Contact Us
Do you want to know more about the CRU Groningen or do you have questions? We are happy to help:
Call us
06-25 65 13 06
Mail us
info@clinicalresearchunitgroningen.com
Visit us
Hanzeplein 1, Triadegebouw Bouwdeel 64, G0 vleugel Groningen